Controlled Release Profiles
Formulated to maintain integrity and effectiveness during storage.
At Credo Life Sciences, we specialize in the manufacturing of high-quality Sustained Release (SR) pellets designed to meet the evolving needs of the global pharmaceutical industry. Our SR pellets are developed with superior dissolution profiles, consistent release kinetics, and customizable formulations that adhere to stringent international regulatory standards including EU, SFDA, WHO guidelines.



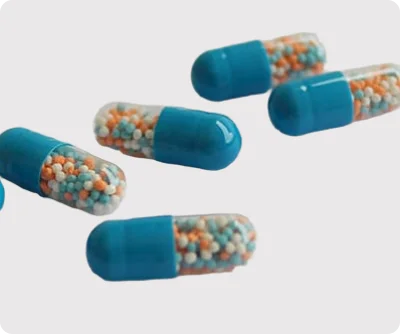
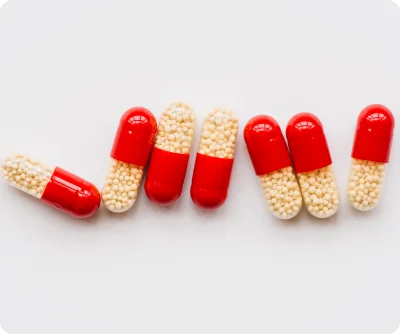
Our Sustained Release (SR) pellets are advanced multiparticulate dosage forms developed to provide controlled and predictable drug release over an extended period. By regulating the release rate of active pharmaceutical ingredients (APIs), our SR pellets help maintain steady plasma concentrations, ensuring consistent therapeutic outcomes and improved patient safety.
We design SR pellets with a strong focus on patient convenience and compliance. By reducing dosing frequency and minimizing drug plasma fluctuations, these formulations not only enhance bioavailability but also lower the risk of adverse effects. This makes them ideal for a wide range of therapeutic applications, from cardiovascular and central nervous system treatments to gastrointestinal and anti-infective therapies.
To achieve the highest level of performance, we utilize cutting-edge manufacturing technologies such as extrusion–spheronization, fluid bed coating, and advanced polymer layering techniques. These processes ensure uniform particle size distribution, excellent flow properties, and robust mechanical stability, delivering consistent quality that aligns with global regulatory standards.

Formulated to maintain integrity and effectiveness during storage.

Manufactured under strict GMP and international compliance norms.
Uniform drug release ensuring reliable bioavailability
Tailored solutions to meet diverse therapeutic needs.
Engineered with precision technology for improved therapeutic outcomes

Optimized formulation ensures better absorption and therapeutic efficiency.
| Product Name | Percentage Available | Therapeutic Usage |
|---|---|---|
| Aceclofenac | 60% | Anti-inflammatory |
| Cinitapride | 1.45% | GI Motility Agent |
| Domperidone | 17% / 20% / 22% / 30% | Anti-emetic |
| Galantamine | 8% | Alzheimer’s Treatment |
| Indomethacin | 27.5% | Anti-inflammatory |
| Itopride | 60% | GI Motility Agent |
| Ketoprofen | 70% | Anti-inflammatory |
| Mebeverine HCI | 80% | Anti-spasmodic |
| Nifedipine | 10% | Anti-hypertensive |
| Nitroglycerin | 1.4%, 2.5% | Anti-anginal |
| Tamsulosin + Dutasteride | 0.4 mg + 0.5 mg | BPH Treatment |
| Tamsulosin HCL | 0.12% / 0.133% / 0.14% / 0.16% / 0.20% | BPH Treatment |
| Venlafaxine HCI | 33%, 50% | Anti-depressant |
You will get End-to-end complete information about our product

Advanced formulations tailored to market needs.

International regulatory compliance

Flexible batch sizes and strength variations

Dedicated support and transparent processes

Trusted pharmaceutical partner across multiple geographies