Advanced Coating
Ensures targeted intestinal drug release.
Our Enteric-Coated (EC) Pellets are designed to protect active pharmaceutical ingredients (APIs) from the acidic environment of the stomach and ensure targeted drug release in the intestine, where absorption is optimal. By preventing premature degradation of APIs, EC pellets play a crucial role in improving drug stability, bioavailability, and therapeutic performance. Enteric-Coated pellets are widely used in developing modified-release formulations, enabling pharmaceutical companies to create patient-friendly dosage forms with enhanced safety and efficacy. They are especially suitable for drugs that are unstable in gastric acid, irritant to the stomach lining, or require release in a specific intestinal region.



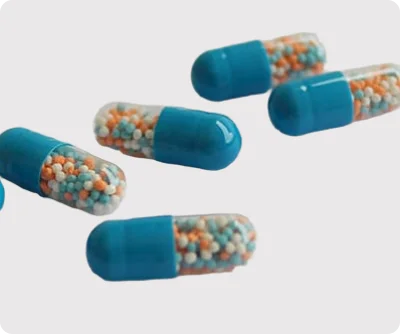
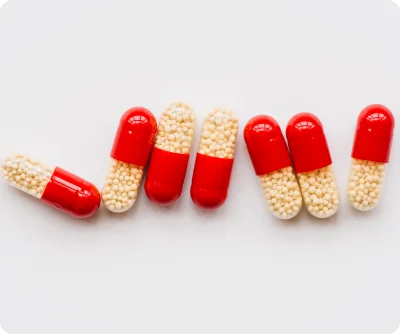
Our Enteric-Coated (EC) Pellets are manufactured using advanced coating technologies and high-performance excipients to ensure precise and predictable drug release. We focus on delivering uniform particle size distribution, accurate API loading, and robust mechanical stability, which guarantees consistency across batches.
By leveraging fluid bed coating, layering, and extrusion-spheronization techniques, we create pellets that withstand gastric conditions while ensuring release at the desired intestinal pH. This makes our EC pellets highly suitable for a wide variety of APIs and therapeutic classes, including proton pump inhibitors (PPIs), antibiotics, anti-inflammatory agents, and cardiovascular drugs.
With a strong emphasis on global regulatory compliance (EU,SFDA,WHO GMP standards), we ensure that our EC pellets meet the stringent quality requirements of regulated and semi-regulated markets. Our expertise lies not only in standard formulations but also in customized development to align with market needs, therapeutic goals, and brand differentiation strategies.

Ensures targeted intestinal drug release.

Protects APIs from acidic degradation.
Uniform drug release ensuring reliable bioavailability
Compatible with capsules, tablets, and sachets.
Manufactured under cGMP and international guidelines.

Maintains accurate API loading and particle consistency.
| Product Name | Percentage Available | Therapeutic Usage |
|---|---|---|
| Aspirin | 40% / 65% | Cardiovascular |
| Choline Fenofibrate | 60% | Lipid Regulator |
| Dexlansoprazole DR | 17% / 20% / 22.5% / 23% / 25% | Anti-ulcerant |
| Dexlansoprazole DDR | 17% / 20% / 22.5% / 23% / 25% | Anti-ulcerant |
| Duloxetine HCl | 9% / 12% / 17% / 20% / 25% | Anti-depressant |
| Esomeprazole Mg | 8.5% / 22.5% | Anti-ulcerant |
| Omeprazole | 23.5% | Anti-ulcerant |
| Pancreatin (Veg/Por) | 60% / 70% | Digestive Enzyme |
| Pantoprazole Sodium | 16% / 20% / 22.5% | Anti-ulcerant |
| Rabeprazole | 12% / 15% / 20% | Anti-ulcerant |
You will get End-to-end complete information about our product

Advanced formulations tailored to market needs.

International regulatory compliance

Flexible batch sizes and strength variations

Dedicated support and transparent processes

Trusted pharmaceutical partner across multiple geographies