Innovative Tech
Advanced IR, SR, MR, and EC formulations.
At our R&D facility, we are continuously developing high-quality pharmaceutical pellets and granules to expand our product portfolio and meet emerging therapeutic needs. Our current pipeline includes APIs in IR, MR, SR, and EC formats, tailored for enhanced bioavailability, patient compliance, and targeted drug delivery. We focus on creating formulations that address global market requirements, including regulatory compliance for Europe, the US, and other international markets. Our offerings include Aprepitant IR Pellets, Choline Fenofibrate EC Pellets, Fenofibrate IR and SR Granules, Methylphenidate IR and MR Pellets, Metoprolol Succinate ER Pellets, Omeprazole EC Pellets, and Pancreatin EC Pellets, among others. We also develop custom taste-masked granules as per customer specifications.



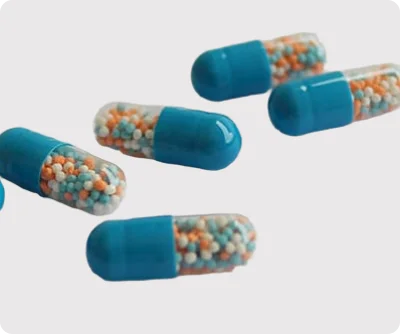
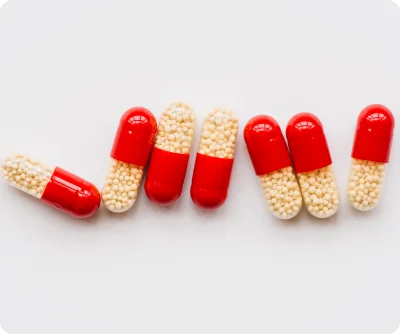
Our pipeline emphasizes innovation, quality, and flexibility. Each product is developed using advanced techniques such as extrusion-spheronization, fluid bed coating, and microencapsulation, ensuring consistent drug release, stability, and patient-friendly formulations. We maintain stringent quality controls at every stage of development to meet international regulatory standards.
We specialize in developing customized solutions, allowing clients to define release profiles, dosage forms, and excipient composition. This flexibility ensures that our products can be tailored for diverse therapeutic applications, including pediatric, geriatric, and adult patient populations.
With a commitment to global market readiness, our R&D team ensures that every formulation is aligned with EU,SFDA,WHO GMP guidelines, providing safe, effective, and commercially viable pharmaceutical products.

Advanced IR, SR, MR, and EC formulations.

Flexible APIs, dosage forms, and release profiles.
Ensures consistent efficacy and outcomes.
Developed under cGMP, USFDA, EU, and WHO-GMP.
Taste-masked, pediatric, geriatric, and multi-API options.

From pilot batches to commercial-scale manufacturing.
| S.No. | Product Name |
|---|---|
| 1 | DILTIAZEM HCL FR PELLETS 56% |
| 2 | DILTIAZEM HCL SR PELLETS 50% |
| 3 | DOXYCYCLINE PELLETS 70% |
| 4 | LACTIC ACID BACILLUS EC PELLETS 5 BILLION SPORES |
| 5 | METHYLPHENIDATE HCl IR PELLETS 13% |
| 6 | METHYLPHENIDATE HCl MR PELLETS 10% |
| 7 | METOPROLOL SUCCINATE ER PELLETS 65% |
| 8 | OMEPRAZOLE EC PELLETS 8.5% (EUROPE) |
| 9 | PANCREATIN EC PELLETS 83% (PORCINE SOURCE) |
| 10 | PANCREATIN EC PELLETS 85% (PORCINE SOURCE) |
| 11 | PAPAIN PELLETS 60% |
| 12 | PREGABALIN IR PELLETS 25%, 50% & 75% |
| 13 | SECNIDAZOLE GRANULES 80% |
| 14 | THIOCOLCHICOSIDE SR PELLETS 8% |
You will get End-to-end complete information about our product

Advanced formulations tailored to market needs.

International regulatory compliance

Flexible batch sizes and strength variations

Dedicated support and transparent processes

Trusted pharmaceutical partner across multiple geographies