Compliance
DMF-registered, meeting USFDA, EU, and WHO-GMP standards.
Our Drug Master File (DMF) registered pellets are designed to meet the regulatory requirements of international pharmaceutical markets. These pellets are developed with high-quality APIs and excipients to ensure safety, efficacy, and consistent performance across various dosage forms. We maintain DMF documentation to support regulatory filings in the Europe, and other global markets, providing pharma companies with reliable data on formulation, manufacturing process, and quality control. Our DMF-registered products cover IR, SR, MR, and EC pellet formulations, enabling faster market approvals and streamlined commercialization.



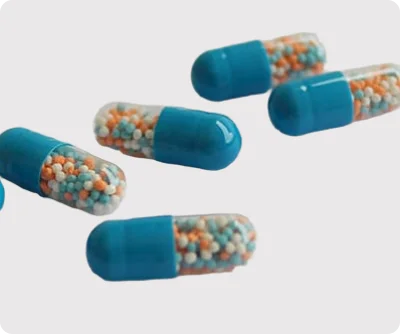
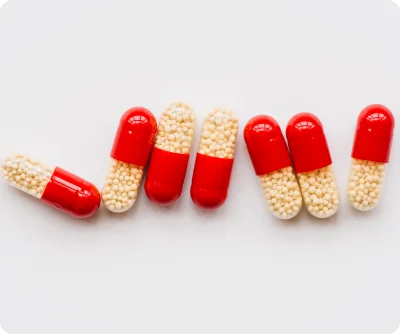
Our DMF-registered pellets are developed under stringent cGMP and international quality standards, ensuring regulatory compliance at every stage of production. Each batch undergoes rigorous quality testing, stability assessment, and documentation, making it suitable for submission to regulatory authorities.
We provide full transparency and technical support to clients using our DMF pellets, including composition, manufacturing process details, analytical methods, and stability data. This ensures that pharmaceutical companies can rely on our products for formulation development, clinical trials, and commercial production.
By offering customized pellet solutions, we help clients design therapeutic formulations tailored to specific release profiles and patient needs, all while maintaining adherence to regulatory requirements and market expectations.

DMF-registered, meeting USFDA, EU, and WHO-GMP standards.

Consistent particle size, purity, and stability.
Supports IR, SR, MR, ER, and EC pellets.
Flexible APIs and release profiles.
Taste-masked and suitable for all age groups.

From pilot batches to commercial-scale manufacturing.
| Product Name | Therapeutic Usage | Quality Standard | DMF Status |
|---|---|---|---|
| Azithromycin Taste Mask Granules 7.5% | Anti-Bacterial | USP | DMF AVAILABLE |
| Dexlansoprazole DDR 20% | Anti-Ulcerant | IH | DMF AVAILABLE |
| Dexlansoprazole DDR 25% | Anti-Ulcerant | IH | DMF AVAILABLE |
| Duloxetine HCl EC 17% | Anti-Depressant | USP | DMF AVAILABLE |
| Duloxetine HCl EC 20% * | Anti-Depressant | USP | DMF AVAILABLE |
| Dutasteride IR 0.5% | Diuretic | IH | DMF AVAILABLE |
| Esomeprazole Magnesium EC 8.5% | Anti-Ulcerant | USP | DMF AVAILABLE |
| Esomeprazole Magnesium EC 22.5% | Anti-Ulcerant | USP | DMF AVAILABLE |
| Itraconazole IR 20% | Anti-Fungal | BP/USP | DMF AVAILABLE |
| Itraconazole IR 22% | Anti-Fungal | BP/USP | DMF AVAILABLE |
| Itraconazole IR 30% | Anti-Fungal | BP/USP | DMF AVAILABLE |
| Itraconazole IR 40% | Anti-Fungal | BP/USP | DMF AVAILABLE |
| Mebeverine HCl IR 40% | Anti-Spasmodic | IH | DMF AVAILABLE |
| Mebeverine HCl SR 80% | Anti-Spasmodic | IH | DMF AVAILABLE |
| Nitroglycerin SR 1.4% | Anti-Anginal | IH | DMF AVAILABLE |
| Nitroglycerin SR 2.5% | Anti-Anginal | IH | DMF AVAILABLE |
| Orlistat IR 50% | Anti-Obesity | USP/IH | DMF AVAILABLE |
| Rabeprazole Sodium EC 15% | Anti-Ulcerant | IH | DMF AVAILABLE |
| Tamsulosin HCl SR 0.12% | BPH | USP | DMF AVAILABLE |
| Tamsulosin HCl SR 0.16% | BPH | USP | DMF AVAILABLE |
| Tamsulosin HCl SR 0.20% | BPH | USP | DMF AVAILABLE |
| Tamsulosin HCl SR 0.4 mg + Dutasteride IR 0.5 mg | BPH | USP/IH | DMF AVAILABLE |
| Venlafaxine HCl SR 33% * | Anti-Depressant | USP | DMF AVAILABLE |
You will get End-to-end complete information about our product

Advanced formulations tailored to market needs.

International regulatory compliance

Flexible batch sizes and strength variations

Dedicated support and transparent processes

Trusted pharmaceutical partner across multiple geographies